


StitchKit comes pre-loaded in various suture combinations for maximum ease-of-use. Suture options include barbed, absorbable, non-absorbable, monofilament, and braided.
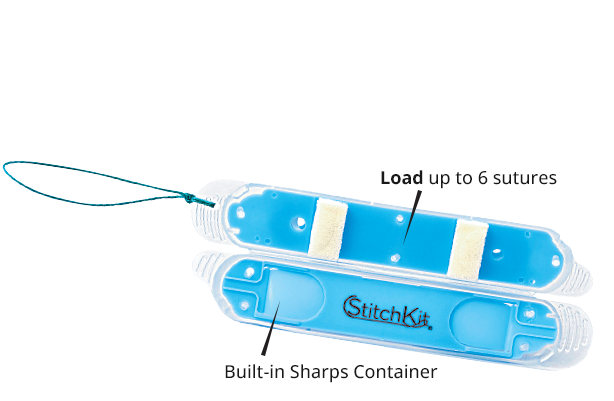

StitchKit PARK comes unloaded for maximum flexibility, allowing up to 6 sutures of any type to be loaded on the back table and deployed into the surgical field.


All StitchKit configurations have an on-board sharps container to ensure that needles are secure throughout the case.
Ordering Info
Click here for ordering information.
FAQs
Please see our FAQ page.
Try our Value Analysis Tool to see how much value StitchKit can bring to your practice.